Mixing of Sterile Ingredients
To respond to legislative and customer requirements, the pharmaceutical industry is having to demand ever increasing standards of hygienic construction in process equipment. This is applicable to all forms of pharmaceutical products - ointments, suspensions, syrups, etc. Products manufactured under sterile or cleanroom conditions - for example injectables and vaccines - present different challenges, especially regarding product sterilisation. For these products, mixing would normally take place before sterilisation but there are some instances where post-production sterilisation is impossible for technical reasons, placing further restrictions on the mixing process. Often products will be subject to a combination of these factors:
- When the finished product is temperature sensitive and cannot be heat sterilised.
- Where irradiation, often used to sterilise heat sensitive products, can damage the product.
- Where the product is a suspension and cannot be sterilised by filtration.
The Process
In addition to the demands of the actual mixing task, process equipment must meet a number of requirements, in terms of both design and construction:
- Equipment must be designed to be Cleaned-In-Place (CIP) and Sterilised-In-Place (SIP).
- Any mixing device must have as near to zero retention as possible.
- Dead areas and crevices (“bug traps”) must be eliminated.
- Materials of construction, including all product contact parts, must be from product compatible and traceable sources.
- Equipment may be required to conform to standards such as FDA, EHEDG and cGMP.
- Detailed documentation - for example packages to comply with FDA regulations - may also be required.
The Problem
Conventional agitators which are modified to be hygienic and magnetic stirrers have limitations. These are regarded as process aids and are suitable for optimising heat transfer and simple liquid/liquid mixing.
- These low shear devices are not suitable where more complex mixing duties are involved, e.g. creating emulsions or modifying rheology/viscosity.
- Magnetic stirrers are less appropriate for viscous materials and suspensions due to their design.
The Solution
Silverson has developed a range of Ultra Hygienic mixers suitable for pharmaceutical and biotechnology applications, including processing of sterile ingredients. This technology is detailed in the following section. Silverson can also assist in the preparation of Installation Qualification/Operational Qualification protocols for process equipment (IQ/OQs) and other documentation including data dossiers for FDA and other regulatory body validation.
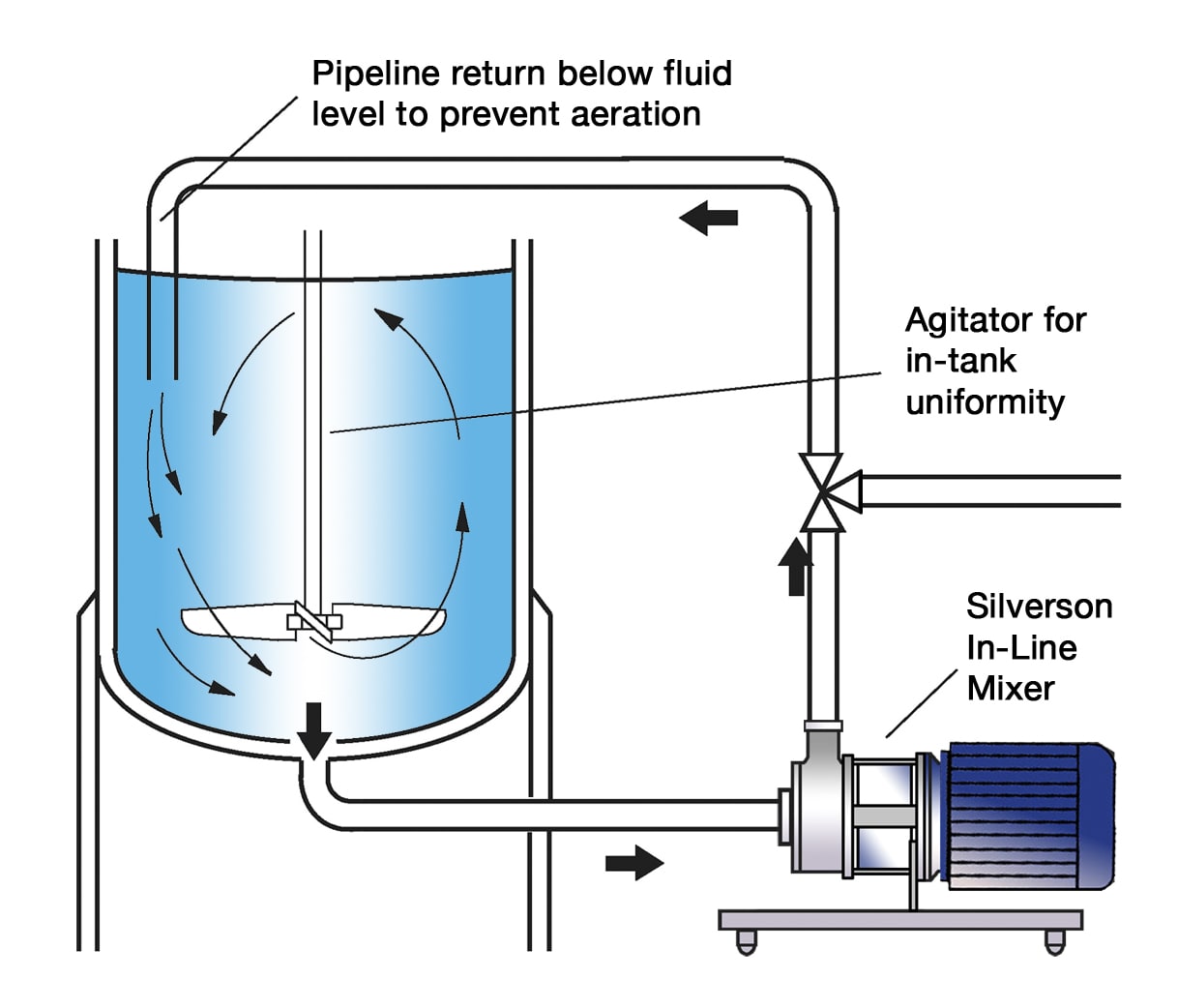
UHS Ultra Hygienic In-Line Mixers
All the qualities and flexibility of standard In-Line mixers plus additional hygienic features and options:
- Certified by both 3A and EHEDG
- Constructed from materials on the FDA master list
- Designed for Clean-In-Place and Sterilise-In-Place operation
- Self-draining tangential outlet
- Single piece inlet plate and stator (optional)
- Ultra Hygienic shaft sealing
- All product contact parts in 316L stainless steel
- Standard machine finish to 0.8 µm Ra and optional fine finish to 0.5 µm Ra, both available with surface finish certification
- Electropolished finish also available
- Crevice-free construction
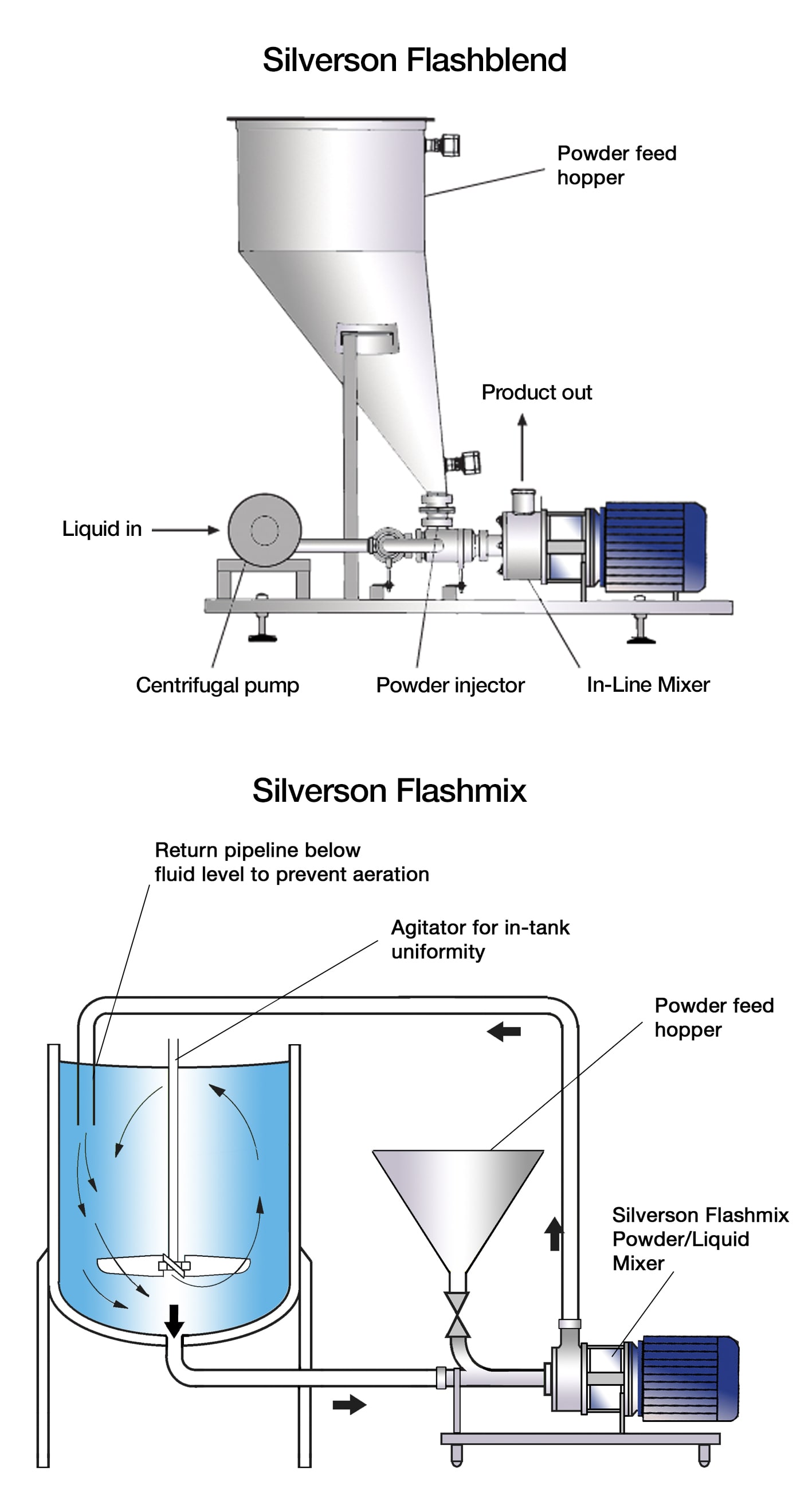
Powder/Liquid Mixers
A number of modifications can be made to the standard models:
Based on an Ultra Hygienic UHS In-Line mixer
- Product contact parts constructed in 316L stainless steel with finishing options as above for UHS In-Line mixers
- The system can be self-draining throughout
- Optional valve types including high specification electropolished diaphragm valves can be used for the powder feed and CIP
- A hygienic design centrifugal pump conforming to the same standard as the In-Line mixer can be used on the Flashblend
- Various measures for dust filters or extraction can be incorporated
- The hopper can be supplied with a hygienic lid and can be modified to accommodate various conveyors and bulk powder dispensing systems such as “bulk bags” and FIBCs
- Where low volume, high cost or hazardous ingredients are being handled, small detachable hoppers can be supplied. This allows loading of powder outside the cleanroom or in a dispensary, minimising powder handling in the clean area
- The hopper and lid can be designed for CIP/SIP or manual cleaning and autoclaving
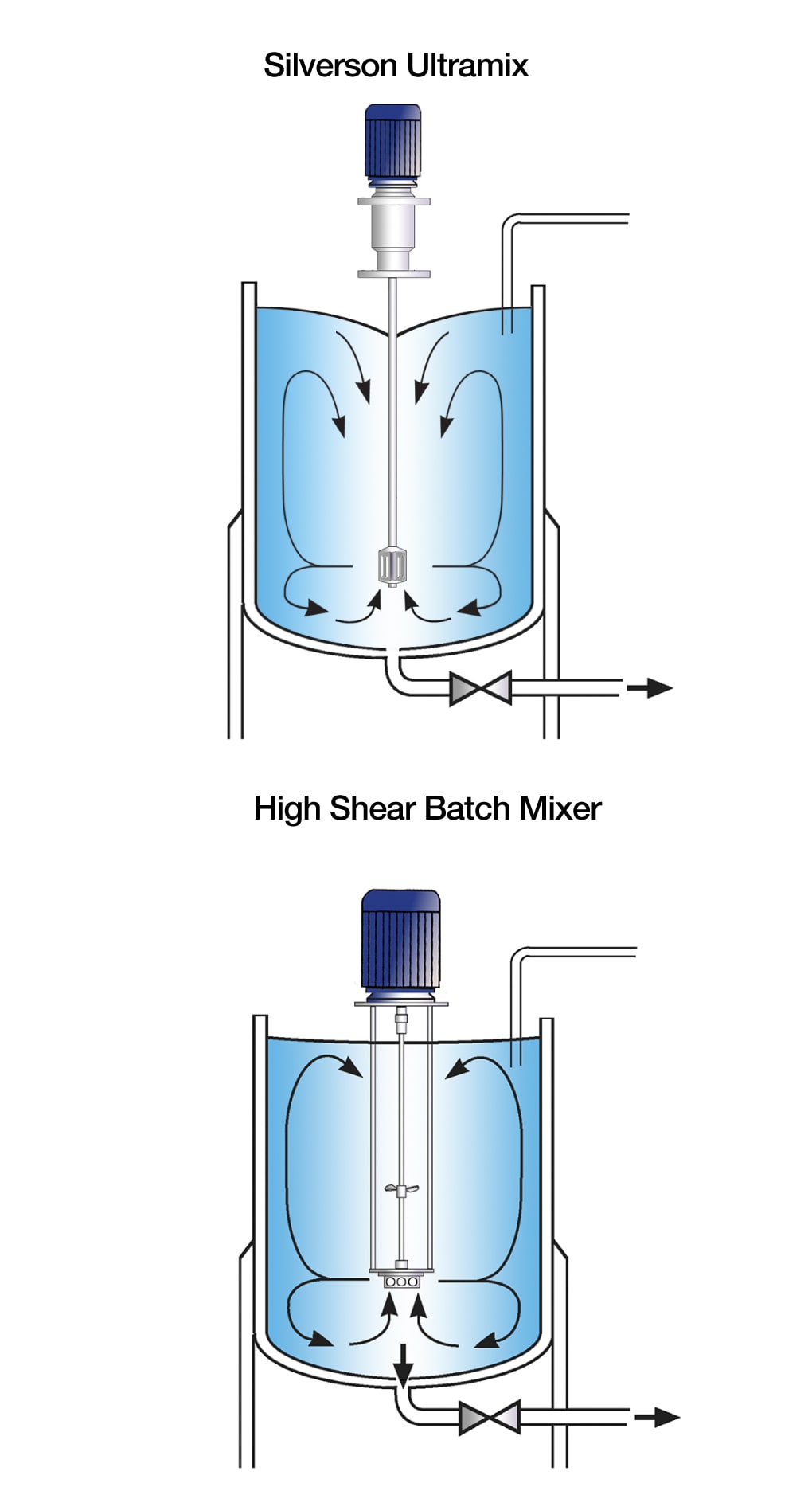
In-tank Mixers
Silverson Ultramix mixers feature a single shaft, single piece mixing head sanitary design ideal for hygienic applications. The Ultramix is designed for Cleaning-In-Place (CIP) with Sterilise-In-Place (SIP) as an option.
High shear Batch mixers can also be offered with hygienic features and optional modifications:
- Typically used for batch sizes of up to 1000 litres
- Clean-In-Place (CIP) design
- Finishing options as above for UHS In-Line mixers
- Ultra Hygienic crevice-free construction
- A range of motor options including explosion proof and compressed air power
- Quick-release motor to permit autoclaving of vessel and mixer
- Depending on model, seals can be product lubricated, lubricated with product compatible liquid or sterile gas flushed
- Mixers can be supplied for operation in sealed vessels under vacuum, atmospheric or positive pressure
- Units can be supplied for use in vessels with relatively small openings
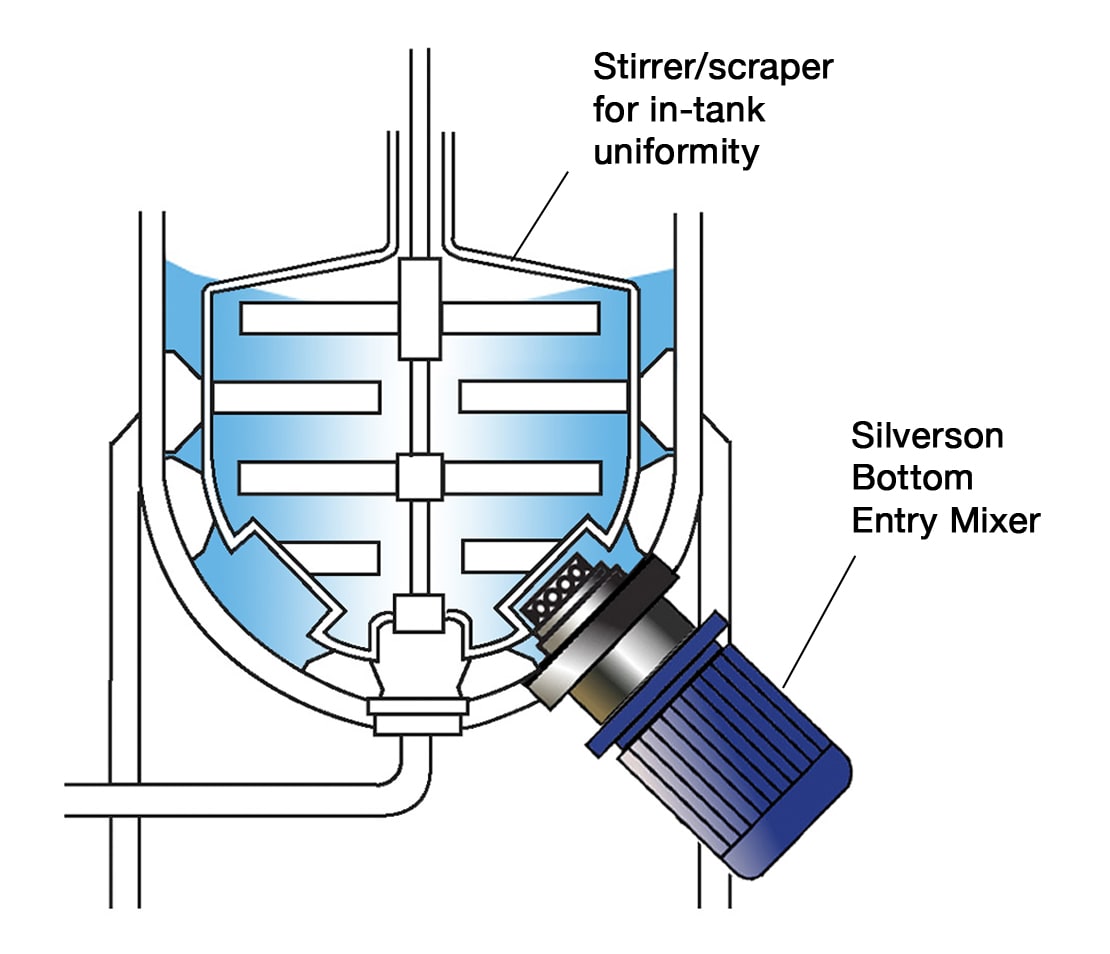
High Shear Bottom Entry Mixers
- Can be designed for Ultra Hygienic applications, with minimised number of product contact parts, crevice-free construction and hygienic shaft sealing
- Finishing options as above for UHS In-Line mixers
- Ideal for hydrating large quantities of powders
- Ideal for products such as creams that increase in viscosity on cooling and where heat sensitive ingredients may only be added at low temperatures
- Normally used in conjunction with a scraper unit